This article is part of a series about how OS Fund (OSF) companies are radically redefining our future by rewriting the operating systems of life. Or as we prefer to think about it: Step 1: Put a dent into the universe. And Step 2: Rewrite the universe. You can see the full OSF collection here and read more about Building a Biological Immune System.
In contemplating the future, I love imagining how our daily lives today will be thought of in the future. What appears sci-fi to us today but will be “normal” 50 years from now? What inefficient and boneheaded things do we do today that future generations will look back and laugh at?
Seeing beyond what’s possible is a rare skill. Being able to design and build beyond what’s possible is even more rare. Put together, this is the unique set of skills and abilities that OSF founders all have in common. Most importantly, they’ve chosen to focus their abilities to tackling the biggest problems humanity faces.
But who are they? What makes them tick? Why do this versus other things? And how might their technologies change the world? I set out recently to learn a little more about the companies I invest in. These are their stories.
I. Introduction: Rare Proteins, Worth Their Weight in Gold
I’d like to start with a small quiz. Take these five facts:
1. The only non-government plane which flew in the U.S. the day after September 11, 2001, went from San Diego to Florida.
2. A “Golden Arm” blood donor in Australia saved an estimated 2.4 million lives.
3. Scientists recently trawled the oceans for ancient bacteria.
4. Doctors across the U.S. scrambled in emergency rooms as summer, also known as “trauma season”, started.
5. Virologists injected chicken eggs with a virus, like they do every year on the dot.
Q: What do these Herculean labors all have in common?
A: Heaven and earth is moved in order to find, capture, or replicate one and only one thing in all five of these scenarios: Rare proteins. All to get the right protein in the right place at the right time.
1. The plane flew in the empty skies after 9/11 because a snake handler had been bitten in Florida and was rapidly dying. The only good vaccine was in California, so the FAA approved the small private plane to fly all the way across the country to save the man’s life. Protein.
2. The “Golden Arm” guy in Australia has a unique protein in his blood which almost no one else has and which is necessary to treat a certain medical condition, so he donates as much blood as he can. Protein.
3. Molecular biologists looking for new types of photosynthesis can in theory design them in labs, but it is today so difficult that it makes more sense to just “steal” highly-evolved and specialized proteins from nature’s wet lab — the ocean. Hence scientists trawling the oceans with nets. Protein.
4. Hospitals across the country are running low on some drugs because of “manufacturing problems” at one of its major pharma suppliers. What are they having trouble making, which modern chemistry can’t quite get perfect? Protein.
5. And those chicken eggs? They are a step in one of the most common ways to make the seasonal flu vaccine, because nothing we can make in the lab can quite mimic the energy and protein-rich environment of a good ol’ fashioned egg. Protein.
My point?
All these efforts — the time, the money — will look crazy in retrospect, if a company I recently invested in, Arzeda, has its say.
Many of today’s medical processes — chemotherapy, vaccine production, blood donation — will look as backwards to future generations as leeches, bloodletting, lobotomy, and trephination do to us now.
I recently sat down with Arzeda co-founder Alexandre Zanghellini, who explained a world where cancer could be targeted by bespoke treatments, where plants are more efficient, and where manufacturing could be done with little to no waste, anywhere in the world.
It seems to me that our ability to computationally design new proteins will be equally important as engineering DNA.
I. Arzeda Hopes to do for Biology what Gutenberg Did For Paper: Invent the Printing Press
Tl;dr: We can design new proteins that would never occur in the evolutionary process, even if evolution was given perfect conditions and infinite time.
We all know that DNA is special because small DNA differences are the differences between all of us. Large differences are the differences between species.
But it’s not actually the DNA that makes us different, it’s what the DNA makes — proteins. (And no, I’m not talking about the powder you put in smoothies, but rather the most basic molecules that make up every cell in living organisms. Almost everything organic and biological is made up of proteins. Every cell, every virus, every person. Proteins.)
Everything that’s alive, whether plant or human, is run by these small nanomachines. If we had better, more efficient proteins to do our biological work for us in fields like agriculture, medicine, and manufacturing, some of the intractable problems threatening our future suddenly become solvable.
The work that Arzeda co-founder Alexandre Zanghellini and his team are doing is prompting a paradigm shift in the way we create and cultivate proteins. Rather than relying on natural selection and serendipity, Arzeda is rewriting the operating system of protein synthesis by asking: How can we make proteins more efficiently than would ever be possible through evolution?
“What we bring is that by computational design, you don’t need to use the fishing net to find the protein in the first place,” said Zanghellini.
Making a new protein in nature is slow: a mutation arises over generations that provides a benefit to the organism, and so it propagates into future generations.
“The ability to design proteins, or change them at will, can impact every living organism. That means we can make better plants that help us solve global warming and improve food production. We can make better therapies that are a lot more specific and tailored. And we can also transform manufacturing because pretty much everything that we make is indirectly, ultimately, made from biology,” Zanghellini told me.
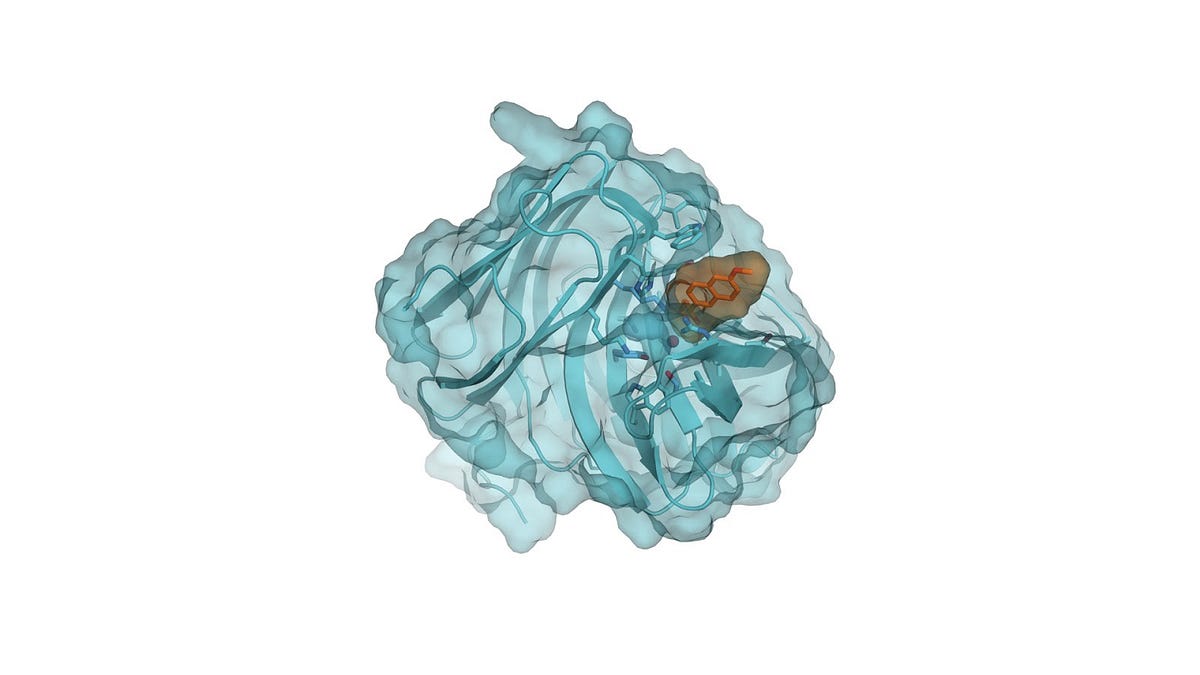
A company would typically spend months or even years scouring the far corners of earth, from the Arctic to the Amazon, gathering thousands of samples of animals and plants, culturing them, screening possible candidates, subjecting the best bets to random mutations, then screening some more. This was the state-of-the art in the 20th century, just looking and hoping.
The old methodologies are extremely risky from an investment standpoint. They required dozens of people working around the clock, resulted in 98% false positives at best, and were in general incredibly expensive.
“Biology, and even synthetic biology, today still uses the approach of early humans, which is, ‘I’m just going to go around and pick whatever nature has to offer, and come back and see if I can make something that works.’ But progress comes when we carve our own tools. We didn’t get where we are by just using the tools and the stones that we found in nature. What got us out of living in a cave was the ability to manufacture our tools for the job, whether it was hunting, or cutting, and it’s exactly right. We are rationally cutting the best tool for the job, not relying on what nature has provided us.”
“In the future, I think people will look back at the techniques that we used, that were used today, and say, how could we ever get anything done that way?”
II. How to Print Proteins
Arzeda is moving past the evolutionary process by designing better software tools to do the heavy lifting of design, chemistry, and synthesis.
“It’s the difference between hacking the system versus designing a new system from scratch,” Zanghellini said.
At Arzeda, they use software to simulate new proteins, greatly reducing the time and energy required to sift through nature’s versions. It also helps with production. Chemistry is hard, and synthesizing proteins is not 100% every time. But Arzeda uses their pipeline to increase efficiency for error-free synthesis to 98 or 99 percent. They’ve honed in on a much better way to make chemistry happen.
“We understand the physics, and we use that physics and the generative potential of algorithms to create artificial proteins that nature would probably never evolve — or that any evolution-based scientific technique would not produce,” said Zanghellini.
With new proteins, we can make better technology in all spheres of life. We may forget that living systems are involved in everything we do and use, sometimes staring us right in the face.
“Because the proteins we design control the molecular details, we are able to get the right molecule nearly 100 percent of the time — and without requiring additional energy,” Zanghellini explained.
“Chemists actually, forever, have been trying to replicate what nature does, which never really worked all that well. So the way we see it is, Why spend the time mimicking what nature does? Why use the same tools as nature? Let’s take the molecules that nature makes, but redesign them so that they’ll do what we want.”
III. What Can We Do With Designer Proteins?
Example 1: More Efficient Plants
Tl;dr: “The ability to design proteins, or change them at will, can impact every living organism.”
Plants are humanity’s lifeline on Earth, providing directly or indirectly the food we eat and the air we breathe. If we want to sustain population growth long into the future, we will almost certainly have to make better plants. Arzeda is working on this from multiple angles.
I asked him to get a little sci-fi. What did he mean, improve plants? Make them taller, faster, stronger?
He was thinking much bigger than that.
“You can imagine that in 20 years from now, or even 10 years from now, we will have plants that capture carbon (CO₂) from the atmosphere much much better, but can produce more food with less fertilizer,” Zanghellini speculated.
One target is RuBisCO, which captures CO₂ from the air (technical term is “fixate”) so that they can make energy and release oxygen into the air. RuBisCo is the most abundant protein on earth, partly because it is so inefficient.
Crazily enough, it is inhibited by oxygen, which was not very abundant in the atmosphere when the protein evolved. To overcome this inhibition, up to 40% of plant biomass is RuBisCo. (Estimates put up to 40 million tons of RuBisCo in the biosphere, about 5kg per person on earth). This is ancient tech folks! And it’s in every plant on our planet.
“How could we improve the key protein that fixates the CO₂? How can we improve, make a new protein, that provides a resistance to drought? And, when we design these, how can we put them into plants in a directed way — an engineering way — as opposed to this serendipitous way of nature?” said Zanghellini
If we could, for example, make a RuBisCo 2.0, we could immediately produce modified plants more able to take advantage our current atmosphere. Plants which would be able to grow faster with the same amount of resources or less.

Previous techniques to modify plants seem primitive even to today’s standards: take a plant, irradiate it, try to cross breed it, and hope to find the trait. What Arzeda is doing is using machine learning to create a new and improved protein to replace RuBisCO altogether. (They’ve already used similar techniques to engineer proteins that can improve corn so that farmers use less herbicide and can obtain higher yields)
Example 2: Sidestepping Oil: A Revolution in Manufacturing
Tl;dr: Everything is made from a small amount of ‘building block’ chemicals that are made from oil. A huge percentage of our entire manufacturing ecosystem is based on oil. Arzeda could one day break this dependence.
In the long term, Zanghellini believes we will not see large buildings with smoke billowing into the atmosphere like we see today — they will have all disappeared, because companies like Arzeda and others in the OS Fund family will have found better ways to produce chemicals.
“Sometimes we forget that oil is a biological product, and we don’t realize how much of the materials around us are actually made from oil,” he pointed out. 5–8% of every barrel of oil extracted end up making the plastics in our car dashboards, nylon in our running gear, LCD displays, and even the gum we chew — it’s all dependent on the synthetic chemistry of the 19th century and, crucially, on the availability of oil.
But in the future with Arzeda’s neo-proteins to direct chemical synthesis, we won’t need natural oil as an input.
“The way we make chemicals would be just a couple of flash fermenters that would look like what you see when you go into Iowa or somewhere in the middle of a country, just these nice little silos, and that’s pretty much it,” Zanghellini speculated.
“All the chemistry, all the compounds, all the materials that you have in your life would be made that way. No more smoke, no more vapor, no more complex chemical processes, because everything would be actually done in the cell, or by proteins that were designed. You would just enter what you want to produce into your computer.”
This could actually change the pace of the world. And in the future, people will look back and say: “Can you imagine when we used to have to drill several miles under the ground just to extract something in its raw form, then take that thing and subject it to high temperature and pressure, just to get a simple molecule?”
The world’s manufacturing system could be entirely microbe-based, or at least microbe-inspired!
That’s why organizations and companies like DARPA, INVISTA, Mitsubishi, and Amyris have all already invested in its ability to rapidly design new enzyme pathways.
Arzeda is bringing the manufacturing industry into the future, and the future to the manufacturing industry.
to learn more go to https://medium.com/future-literacy

2 Pingbacks