Lipofuscin is a waxy heterogeneous substance that is comprised of aggregates of metabolic cellular waste end products, which reside within the lysosomes and cytosols of particular types of human cells. This substance can neither be degraded further via the typical complement of lysosomal enzymes, nor ejected from cells, but can be somewhat diluted through cell division and subsequent cellular growth. This autofluorescent pigment is considered to be a significant aging-associated “wear-and-tear” compound that is found in the liver, kidney, heart muscle, adrenals, nerve cells and ganglion cells. It is specifically arranged around the cell nucleus, and is a type of lipochrome.
In a broader context, lipofuscin is often considered as one of several forms of undesirable proteinaceous agglomerations; others sometimes called ceroids, inclusion bodies, plaques, or aggresomes, contingent on their source and composition. It accumulates over time within aging cells to become manifest as a significant percentage of a cell’s internal volume. As a consequence, it may impede normal cellular processes, affecting proteasomal activity and protein turnover, and is present as typical feature in neurodegenerative diseases as well as aging. Within the aging human brain, deposits of lipofuscin are not uniformly distributed, but rather, are concentrated at specific sites of functional interest. The prevailing thought is that the major source of lipofuscin is derived from the incomplete lysosomal degradation of cell membrane lipids and damaged mitochondria.
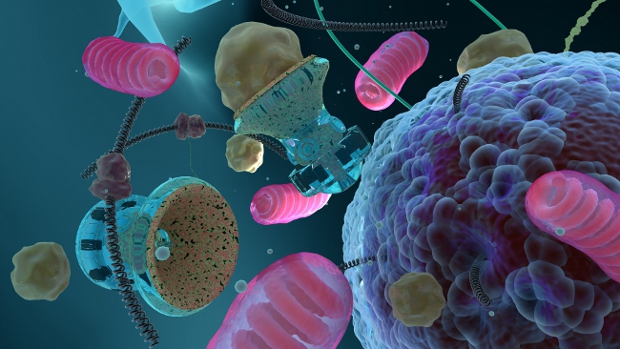
It is conceivable that the future development of nanomedical robotics, as described in the upcoming book: Nanomedical Device and Systems Design: Challenges, Possibilities, Visions (FJ Boehm – Editor), to be published on Nov/22/13 by CRC Press (Taylor & Francis) might enable the capacity for the therapeutic removal of lipofuscin from individual cells in massively parallel fashion without the initial use of UV transparency. Conceptual dedicated autonomous nanodevices (~200 nm in diameter – where one nanometer is a billionth of a meter) might penetrate the cell membranes of neurons and other cells and undertake the removal of lipofuscin through various means, as described in the book excerpt below:
“Although entirely conceptual and seemingly impracticable currently (2013), advanced AI [Artificial Intelligence] involvement in the design/optimization of sophisticated nanomedical devices working in conjunction with mature nanomanufacturing may indeed bring about much more complex, albeit far more rapid and efficient lipofuscin detection and removal. Advanced autonomous nanodevices might precisely locate lipofuscin granules by exploiting its strong fluorescence signatures (emission spectrum ranges from 450 to 700 nm) [1] …. to match with onboard reference spectral profiles. The prospective armamentarium at the disposal of these autonomous diamondoid “defuscin” class nanodevices…. might allow for the complete eradication of lipofuscin aggregates utilizing a feedthrough digestive strategy. These entities may be propelled by arrays of oscillating piezoelectric “fins” or via integrated magnetic nanoparticles, which might be activated and controlled externally. The conical inlet port of the nanodevice would be lined with molecules that possess high affinities for A2E [a primary lipofuscin constituent] and other lipofuscin elements. Once a lipofuscin granule has been captured it would proceed to be drawn into the core, where it would be digested by potent encapsulated enzymes or nanomechanically minced into a liquid state and subsequently purged from the outlet port. This functionality would be similar to Freitas’s microbivore artificial mechanical phagocytes, which operate under a “digest and discharge” protocol” [2].
“The desired result of this defuscin mediated digestion would be the fragmentation and ideally dissolution of the chemical crosslinks that provide lipofuscin its refractive properties. Other defuscin-class nanodevice designs may include proboscises that serve dual purposes; as potential electrodes for highly localized hyperthermic interventions, following insertion into the lipofuscin mass, or hollow nanosyringes… for the injection of powerful cleaving enzymes. The nanomechanical segmentation or disassembly of individual lipofuscin granules at atomic/molecular resolution may be possible employing arrays of diamondoid “debriders” to reduce lipofuscin to its most elemental and harmless fractions. Larger fragments could subsequently be encapsulated for egress through the urinary or gastrointestinal tracts.
The overarching premise here is that treated cells might be thus induced to a more robust state of health (perhaps functioning at more youthful levels) when this cellular burden is extricated.”
Anatomy of a conceptual “defuscin” class lipofuscin removal nanodeviceAnatomy of a conceptual “defuscin” class lipofuscin removal nanodevice
References:
[1] Eichhoff, G., Garaschuk, O., Two-photon imaging of neural networks in a mouse model of Alzheimer’s disease. Cold Spring Harb Protoc. 2011(10), 1206-16, 2011.
[2] Freitas Jr., R.A., Microbivores: Artificial Mechanical Phagocytes using Digest and Discharge Protocol, J. Evol. Technol. 14, 1-52, 2005.
This piece will be appearing in:
Nanomedical Device and Systems Design
This book endeavors to explore a significant range of conceptual nanomedical components, devices and systems, as well as to present some of the latest real world laboratory-based research, which may lead to the development of advanced and highly efficacious nanomedical technologies. This volume is trisegmented; the initial segment utilizes an envisaged conceptual exemplar nanodevice and system (Vascular Cartographic Scanning Nanodevice – VCSN), which the author has evolved, to explore various potential design considerations, which might enable specific functionalities of sophisticated autonomous nanomedical devices. The second segment is comprised of chapters that have been contributed by world class experts in the field, who present and articulate pioneering laboratory-based nanomedical research. The final segment investigates more highly conceptual nanomedical possibilities and visions relating to the implementation of advanced nanomedicine in remote regions and the developing world, as well as nanomedicine in space applications, human augmentation and longevity.
Nanobotmodels Company, in collaboration with the editor of Nanomedical Device and Systems Design: Challenges, Possibilities, Visions (FJ Boehm), have created artistic representations of a conceptual advanced “defuscin” (lipofuscin eradication) nanodevice.
Nanobotmodels was established in 2007 with the goal of developing highly innovative, digital graphics to depict actual and conceptual technologies via the synergistic fusion of art and science. The still nascent, yet prospectively powerful discipline of nanotechnology is poised to radically transform medicine, engineering, biotechnology, electronics and myriad other sectors in the relative near-term. Hence, visionary artistic renderings that portray various aspects of this exciting nanofuture will be beneficial in facilitating a clear understanding of its fundamental concepts to a broad demographic.
Nanobotmodels generates imaginative and engaging state-of-the-art nanotechnology and nanomedical illustrations and animations. Any prototypical component, device, system or far flung concept that might be conceived of can be translated into captivating and colorful photorealistic animated or static renderings and presentation materials…. We bring them all to life for you!
by Y. Svidinenko and FJ Boehm
hero image used from http://www.nanobotmodels.com/?page_id=25/404
February 19, 2015 at 1:20 am
archived comments:
“comprised of aggregates of metabolic cellular waste end products”
Including in tears and mucus- correct?
By Alan Brooks on May 04, 2013 at 8:34pm
You might find this link more than interesting http://www.thoughtware.tv/videos/watch/2542-Unfocused-Pulsed-Lasers-Selectively-Destroy-Lipofuscin
By Damian Poirier on May 06, 2013 at 4:40pm
Interesting. However, it’s not clear whether there is a power density threshold beyond which such a technique loses its effect. If there is no threshold then IR light could be used through the skull. It is quite transparent at the wavelengths mentioned. From an evolutionary POV why the skull should be so transparent to IR is interesting.
By Dirk Bruere on May 06, 2013 at 4:49pm
February 19, 2015 at 2:32 am
David,
Ha, you beat me to it! Good job. 🙂